Nothing — Advancing Life
Sciences Together
Hope and Health
Pioneering Excellence in Research
Our Journey
The Year 2023 is a defining milestone on our journey so far. It marks the 15th anniversary of PBRI resilient growth story and its collaborative support brings into ZES Bioscience Pvt. Ltd a trusted R&D and manufacturing partner to the global life sciences industry.
We at ZES Bioscience Pvt. Ltd. know that your job is critical, urgent, and has a direct influence on people's lives. Our mission, 'In every molecule is the promise for improved health,' drives us to ensure the success of our projects, so that we may translate hope into health for millions of people across the world. Our service approach is Explore everything, Miss nothing.

Our Mission
Our Core Values
- Provide excellent client experiences.
- Provide insightful problem solving.
- Be Humbly Confident and Get It Done.
Our Vision
Our Gallery







OUR PRODUCTS
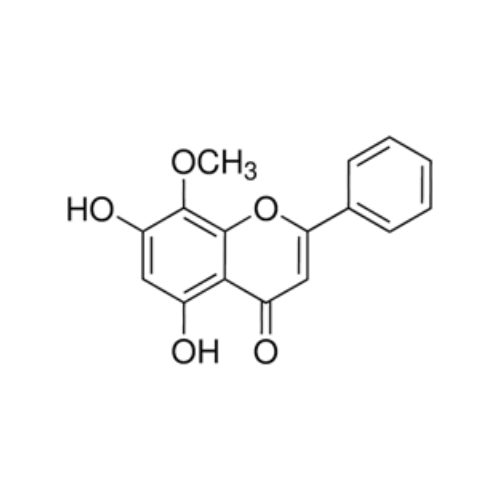
WOGONIN
₹27,463.03 Original price was: ₹27,463.03.₹13,731.00Current price is: ₹13,731.00.
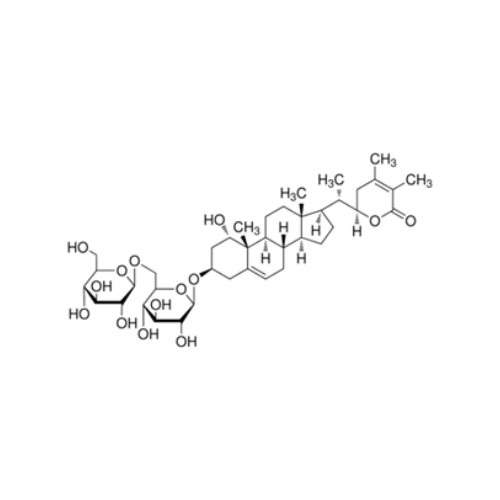
WITHANOSIDE IV
₹56,668.88 Original price was: ₹56,668.88.₹45,335.00Current price is: ₹45,335.00.
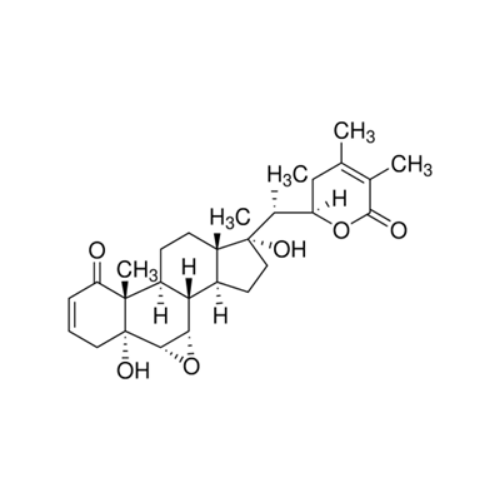
WITHANONE SOLUTION
₹53,080.00 Original price was: ₹53,080.00.₹42,464.00Current price is: ₹42,464.00.
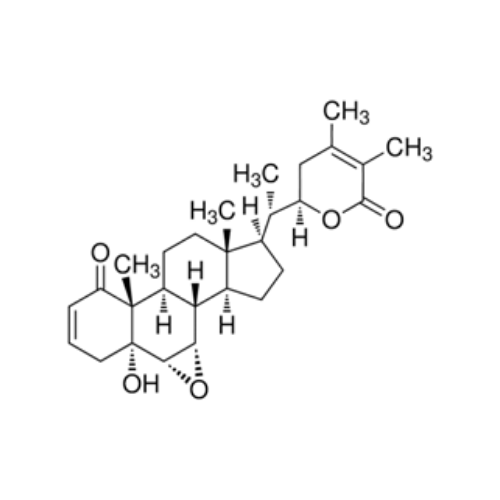
WITHANOLIDE B
₹85,647.40 Original price was: ₹85,647.40.₹68,517.92Current price is: ₹68,517.92.
Our Certification, Grant & Collaboration